Sincalide
For Injection, 5 mcg
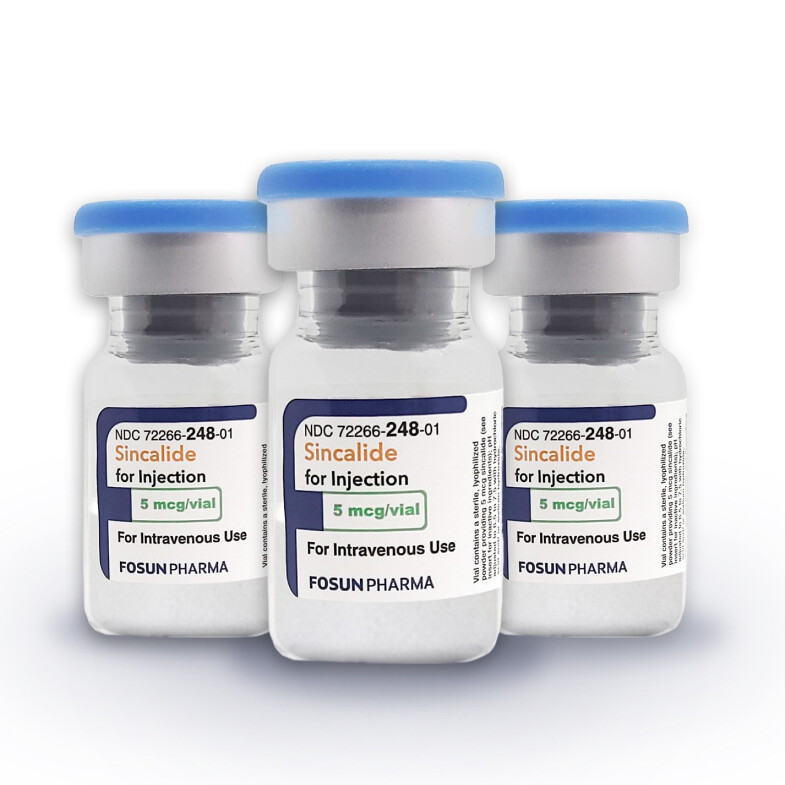
Only FDA Approved Sincalide Product with 24 Months Shelf Life.
Now available and shipping to all 50 states.
Fosun Pharma's Sincalide for Injection provides you with options that may have been unavailable for a long time.
Now with its Current availability and Consistent supply, Sincalide allows you to avoid the inconsistencies of a fatty meal. 1,2
- 24 Months Shelf Life
- Preservative free
- Polysorbate free
- Container closure is not made with natural rubber latex
- Available
References
1. Ziessman, Harvey A., Sincalide: A Review of Clinical Utility, Proper Infusion Methodology, and Alternative Cholecystogogues. Journal of Nuclear Medicine Technology, September 2019, 47 (3) 210-212.
2. Fotos, Joseph S., Tulchinsky, Mark, Oral Cholecystagogue Cholescintigraphy, A Systemic Review of Fatty Meal Options. Clinical Nuclear Medicine, October 2015, 40 (10): p 796-798.
Indications
Sincalide for Injection is a cholecystokinin (CCK) analog indicated in adults to:
- Stimulate gallbladder contraction, as may be necessary by various methods of diagnostic imaging, or to obtain by duodenal aspiration a sample of concentrated bile for analysis of cholesterol, bile salts, phospholipids and crystals.
- Stimulate pancreatic secretin in combination with secretin prior to obtaining a duodenal aspirate for analysis of enzyme activity, composition, and cytology.
- Accelerate the transit of a barium meal through the small bowel, thereby decreasing the time and extent of radiation associated with fluoroscopy and x-ray examination of the intestinal tract.
Important safety information
Warnings and precautions
- Anaphylaxis, Anaphylactic Shock and Other Hypersensitivity Reactions: May occur during or soon after administration. If symptoms occur, discontinue the drug.
- Evacuation of Gallstones: Stimulation of gallbladder contraction in patients with small gallbladder stones could lead to the evacuation of the stones from the gallbladder, resulting in their lodging in the cystic duct or in the common bile duct.
- Gastrointestinal Adverse Reactions with Intravenous Injection: Administration as an intravenous injection may cause transient nausea, vomiting, abdominal pain or cramping, dizziness or flushing. To reduce the risk of adverse reactions when used to simulate contraction of the gallbladder or accelerate transit of a barium meal through the small intestine, administer as an intravenous infusion over 50 or 30 minutes, respectively.
- Preterm Labor or Spontaneous Abortion: Advise pregnant women of the potential risk for preterm labor and spontaneous abortion.
Adverse reactions
Most common adverse reactions (≥20%) are: abdominal discomfort or pain, and nausea.
Drug interactions
Drugs that Affect Gallbladder Motility or Contractile Response: May interfere with response to Sincalide. Consider discontinuing these drugs prior to administration of Sincalide for Injection, when used to stimulate contraction of the gallbladder.
Please see Full Prescribing Information